Zinc Hydroxide Ions . Visit byju's to understand the properties, structure and its uses. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: the solution and complexation chemistry of zinc ions is the basis for zinc biology. the solution and complexation chemistry of zinc ions is the basis for zinc biology. In living organisms, zinc is redox. zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. In living organisms, zinc is redox. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve;
from pubs.rsc.org
zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. Visit byju's to understand the properties, structure and its uses. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; the solution and complexation chemistry of zinc ions is the basis for zinc biology. In living organisms, zinc is redox. the solution and complexation chemistry of zinc ions is the basis for zinc biology. In living organisms, zinc is redox.
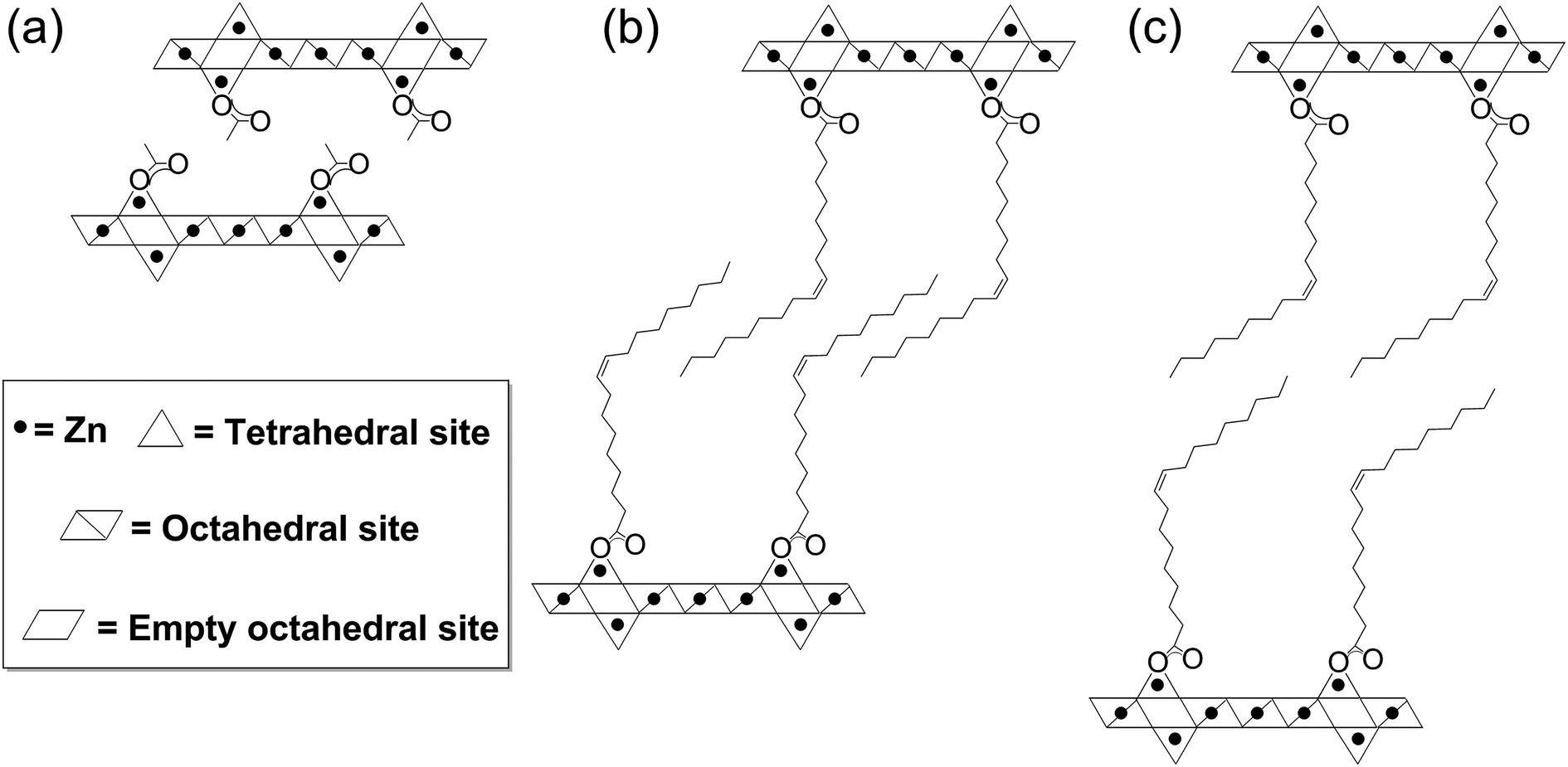
Layered zinc hydroxide monolayers by hydrolysis of organozincs
Zinc Hydroxide Ions In living organisms, zinc is redox. In living organisms, zinc is redox. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; Visit byju's to understand the properties, structure and its uses. the solution and complexation chemistry of zinc ions is the basis for zinc biology. In living organisms, zinc is redox. the solution and complexation chemistry of zinc ions is the basis for zinc biology. zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free Zinc Hydroxide Ions zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. In living organisms, zinc is redox. the solution and complexation chemistry of zinc ions is the basis for zinc biology. Visit byju's to understand the properties, structure and its uses. the solution and complexation chemistry of zinc ions is the basis. Zinc Hydroxide Ions.
From cexjettf.blob.core.windows.net
Copper Zinc Hydroxide at Virginia Martinez blog Zinc Hydroxide Ions since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. the solution and complexation chemistry of zinc ions is the basis for zinc biology. the solution and complexation chemistry of zinc ions is the basis for zinc. Zinc Hydroxide Ions.
From www.gkseries.com
What is formed when zinc reacts with sodium hydroxide? Zinc Hydroxide Ions In living organisms, zinc is redox. zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; In living organisms, zinc is redox. the solution and complexation chemistry of zinc ions is the basis for zinc biology. Visit byju's. Zinc Hydroxide Ions.
From www.nagwa.com
Question Video Understanding Oxidation and Reduction in the Reaction Zinc Hydroxide Ions the solution and complexation chemistry of zinc ions is the basis for zinc biology. Visit byju's to understand the properties, structure and its uses. In living organisms, zinc is redox. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: zinc hydroxide. Zinc Hydroxide Ions.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + Zn(NO3)2 = Mg(NO3)2 + Zn Zinc Hydroxide Ions zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. Visit byju's to understand the properties, structure and its uses. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; In living organisms, zinc is redox. the solution and complexation chemistry of zinc ions is the basis for. Zinc Hydroxide Ions.
From www.numerade.com
SOLVEDA saturated solution of zinc hydroxide, Zn(OH)2, has a pH of 8. Zinc Hydroxide Ions In living organisms, zinc is redox. zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; the solution and complexation chemistry of zinc ions is the basis for zinc biology. Visit byju's to understand the properties, structure and. Zinc Hydroxide Ions.
From en.ppt-online.org
Alkaline Button Cell (Zinc/MnO2 in KOH) online presentation Zinc Hydroxide Ions the solution and complexation chemistry of zinc ions is the basis for zinc biology. In living organisms, zinc is redox. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; Visit byju's to understand the properties, structure and its uses. the solution and complexation chemistry of zinc ions is the basis for zinc biology.. Zinc Hydroxide Ions.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Hydroxide Ions zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: In living organisms, zinc is redox. Visit byju's to understand the properties, structure and its uses. . Zinc Hydroxide Ions.
From pubs.rsc.org
Layered zinc hydroxide monolayers by hydrolysis of organozincs Zinc Hydroxide Ions Visit byju's to understand the properties, structure and its uses. zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. In living organisms, zinc is redox. the solution and complexation chemistry of zinc ions is the basis for zinc biology. In living organisms, zinc is redox. zinc(ii) ion reacts with aqueous. Zinc Hydroxide Ions.
From www.youtube.com
Titration of Zinc Ions with Hydroxide Ions YouTube Zinc Hydroxide Ions the solution and complexation chemistry of zinc ions is the basis for zinc biology. Visit byju's to understand the properties, structure and its uses. the solution and complexation chemistry of zinc ions is the basis for zinc biology. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; In living organisms, zinc is redox.. Zinc Hydroxide Ions.
From www.nagwa.com
Question Video Determining the Chemical Formula of Zinc Hydroxide Nagwa Zinc Hydroxide Ions since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; the solution and complexation chemistry of zinc ions is the basis for zinc biology. In living organisms, zinc is redox. In living organisms, zinc is redox. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Visit byju's to understand the properties, structure. Zinc Hydroxide Ions.
From www.fishersci.com
Zinc Hydroxide, MP Biomedicals, Quantity 100 g Fisher Scientific Zinc Hydroxide Ions In living organisms, zinc is redox. zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. Visit byju's to understand the properties, structure and its uses. the solution and complexation chemistry of zinc ions is the basis for zinc biology. the solution and complexation chemistry of zinc ions is the basis. Zinc Hydroxide Ions.
From www.alamy.com
Zinc hydroxide precipitate formed by adding sodium hydroxide (NaOH) to Zinc Hydroxide Ions In living organisms, zinc is redox. the solution and complexation chemistry of zinc ions is the basis for zinc biology. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; In living organisms, zinc is redox. Visit byju's to understand the properties, structure and its uses. zinc hydroxide is an insoluble hydroxide with formula. Zinc Hydroxide Ions.
From whatsinsight.org
Zinc Hydroxide Structure, Properties, and Uses What's Insight Zinc Hydroxide Ions zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: In living organisms, zinc is redox. In living organisms, zinc is redox. zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; Visit byju's to understand the. Zinc Hydroxide Ions.
From www.slideserve.com
PPT Nomenclature Practice PowerPoint Presentation, free download ID Zinc Hydroxide Ions since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. In living organisms, zinc is redox. the solution and complexation chemistry of zinc ions is the basis for zinc biology. zinc(ii) ion reacts with aqueous ammonia to. Zinc Hydroxide Ions.
From www.researchgate.net
FTIR spectra of zinc hydroxide nitrate intercalated with benzoate (a Zinc Hydroxide Ions since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; Visit byju's to understand the properties, structure and its uses. the solution and complexation chemistry of zinc ions is the basis for zinc biology. In living organisms, zinc is redox. the solution and complexation chemistry of zinc ions is the basis for zinc biology.. Zinc Hydroxide Ions.
From www.numerade.com
SOLVED Consider the insoluble compound zinc sulfide, ZnS. The zinc ion Zinc Hydroxide Ions In living organisms, zinc is redox. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: the solution and complexation chemistry of zinc ions is the basis for zinc biology. zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. the solution and complexation chemistry of zinc ions is. Zinc Hydroxide Ions.
From www.youtube.com
How to Write the Net Ionic Equation for ZnCl2 + NaOH = Zn(OH)2 + NaCl Zinc Hydroxide Ions zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: since the ion is naturally surrounded by water ligands, zinc hydroxide will dissolve; zinc hydroxide is an insoluble hydroxide with formula zn (oh)2 of groups ia and iia elements. the solution and complexation chemistry of zinc ions is the basis for zinc biology. In living. Zinc Hydroxide Ions.